Deep brain stimulation (DBS), currently targeting primarily the anterior thalamic nuclei, is a neurostimulation method that modulates the activity of brain circuits using intracerebrally implanted electrodes, with the goal of reducing the frequency of epileptic seizures.
DBS therapy is intended for adult patients with drug-resistant epilepsy. It is typically considered only after vagus nerve stimulation (VNS) proves insufficient or when VNS implantation is not possible (e.g., due to severe asthma).
At the Epilepsy Center Brno, DBS has been used as a treatment for drug-resistant epilepsy since 2011.
How does the surgery work?
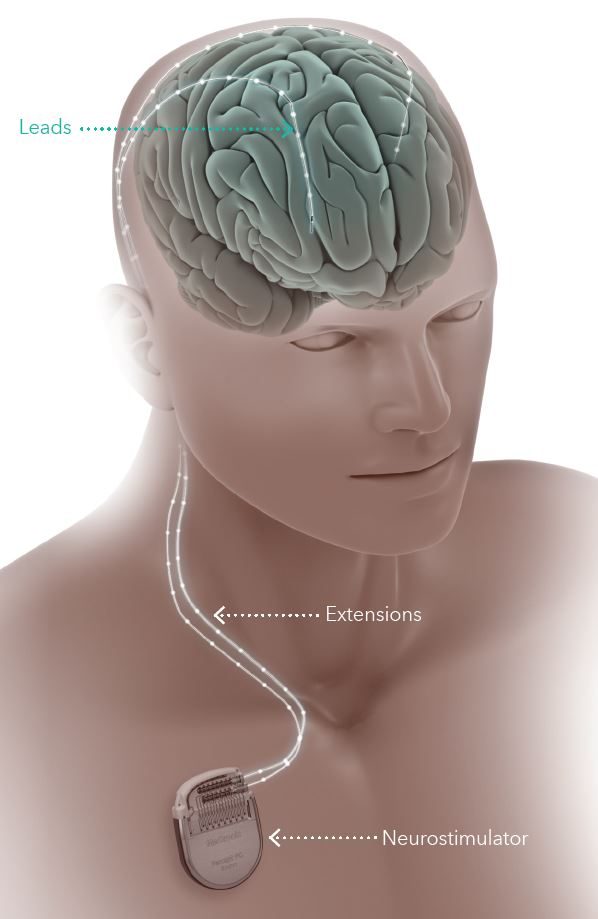
During DBS implantation, stimulating electrodes are first placed into the thalamus, specifically targeting the anterior thalamic nuclei. A battery (neurostimulator) is then implanted under the skin near the collarbone. The battery is connected to the electrodes via a cable that is tunneled under the skin along the neck. The procedure itself is performed under general anesthesia.
How Long Does the Surgery and Hospitalization Take?
You will be admitted to the Department of Neurosurgery prior to surgery. The operation itself lasts approximately 3 to 4 hours. After the procedure, you will remain hospitalized for about one additional week.
What Does Follow-Up Care Look Like?
Several weeks after the DBS stimulator is implanted, neurostimulation therapy is initiated during your first follow-up visit. Your physician will program the DBS system to deliver electrical impulses to your brain continuously—24 hours a day, 7 days a week—in regular cycles.
Finding the optimal settings to reduce seizure frequency while minimizing side effects may take several months. These settings are adjusted gradually and individually during outpatient visits using wireless communication between the stimulator and the physician’s programming device.
DBS stimulation is considered an adjunctive therapy, meaning that you will most likely continue taking anti-seizure medications alongside it. In some cases, a partial or even complete reduction of medication may be possible over time, depending on the effectiveness of stimulation and the judgment of your treating physician.
You will be provided with a patient controller — a handheld programming device about the size of a mobile phone — which allows you to manually activate stimulation if a seizure is approaching, check battery status, or log a seizure event if needed.
When Can I Expect to Feel the Effects?
DBS does not cure epilepsy, but its goal is to reduce the frequency and severity of seizures. The benefits of DBS therapy may not be immediate — it can take up to a year to experience its full effect.
Treatment outcomes vary between individuals. Most patients report a significant reduction in seizure frequency, and some have experienced extended seizure-free periods.
If DBS therapy proves ineffective or the side effects outweigh the benefits, the system can be turned off or surgically removed.
Risks and Side Effects
Some side effects—such as tingling sensations near the implant site or a sudden, short-term increase in seizure frequency or severity after stimulation is initiated—can usually be resolved by adjusting the stimulation settings. Other possible side effects may include abnormal sensations or perceptions, anxiety, confusion, memory disturbances, or depression. Any adverse effects should be promptly reported to your physician.
The implantation of DBS electrodes carries risks similar to any other brain surgery, including infection, fever, discomfort, pain, or swelling at the implant site. All potential risks will be thoroughly discussed with you by your treatment team prior to surgery.


